Chemical physicists investigate the structure and behavior of atoms and molecules on the quantum level. Such research is particularly challenging when the molecule under investigation appears in small amounts and is rapidly transformed into something else, e.g., during combustion, chemical synthesis, or atmospheric chemical reactions. Happily, Research Associate Feng Dong, Fellow David Nesbitt, and former JILAn Scott Davis (now with Vescent Photonics in Denver) have developed an innovative method for studying such elusive chemicals.
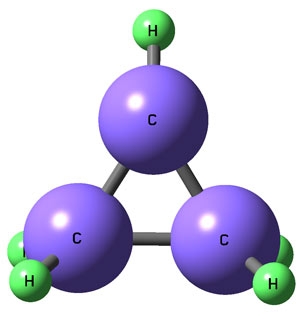
The method combines infrared (IR) laser spectroscopy with slit-jet cooling, which cools the molecules close to 0 K, making them easier to observe. It also uses an electric discharge to create the desired molecule in sufficient quantities for study since elusive chemicals typically exist in vanishingly small amounts. Using the method, the researchers observed the first-ever high-resolution IR spectra for cyclopropyl radical, whose chemical structure is shown on the right. Cyclopropyl radical consists of three carbon atoms in a ring and five hydrogen atoms. It is an important, but short-lived, reaction intermediate in combustion.
The researchers used its IR spectra to determine how the radical's quantum behavior determines the orientation of its hydrogen atoms with respect to particular carbon atoms. They discovered that these orientations change very rapidly - far too quickly for this particular radical to be considered for making certain orientation-specific industrial chemicals. In the future, the new spectral information will allow other scientists to monitor the evolution of cyclopropyl radical during combustion and attempt to discover the mechanisms that create and destroy it. Members of Nesbitt's group will likely explore the radical's behavior in more detail, hoping to determine why its ring structure opens so readily during combustion.
The research discussed here was published online on November 3, 2005, by the Journal of Physical Chemistry A. - Julie Phillips